Peer-to-Peer Realignment
Do our events and content translate into the different kinds of engagements physicians expect today? Is virtual enough for your unique advocate and influencer community?
2021 Health Trends

If there has been one thing that was a sure thing for physicians, it was access to their peers. Nearly all attended in-person events over the course of a year, from advisory meetings to dinners with key opinion leaders, to congresses and conferences. Those ties were abruptly broken when events went virtual or shuttered.
Doctors have filled in that missing peer-to-peer space with ad hoc teleconferences with colleagues and virtual meetups at virtual conferences. For many, something is still missing. As we approach 2021, look for an increasing divide between prescribers and influencers who expect to have face-to-face opportunities again and those waiting out the vaccine, or the end of the virus. One-size-fits all engagements won’t be enough in the years ahead. Peer-to-peer engagement will become much more segmented, increasingly peer-led and demanding of post-Zoom creativity.
Did you know...

75%: In the absence of many industry events, physicians are finding their own ways to connect. 75% reported having daily to monthly ad-hoc teleconferences with peers. 48% are specifically using videoconferencing for that purpose.
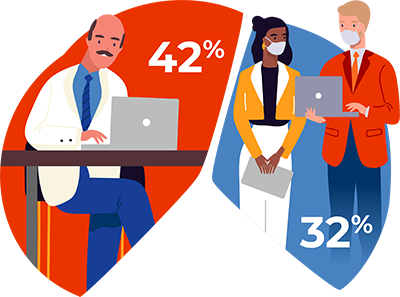
42 vs. 32: In a survey of 250 physicians in the summer of 2020, 42% said they were unlikely or very unlikely to attend in-person networking in the year ahead, assuming a vaccine is not available. However, 32% said they were likely or very likely to go in person. For advisory boards, 47% are unlikely to go in person, but 29% are likely. When thinking about meals with opinion leaders, 49% unlikely, 30% likely.


