サイネオス・ヘルスR&DアドバイザリーEU CTRへの対応状況
EU CTRへの対応はお済みですか?
欧州全体の臨床試験の現状を一変させるため、EU CTR(欧州連合臨床試験規則)が策定されました。新たなCTIS(臨床試験情報システム)が2022年1月31日に正式に稼働し、3年間の移行期間が開始されることとなりました。この期間中に、企業は最新のプロセスを実装し、コンプライアンスとCTISとの効果的な連携が可能かどうか、システムを評価する必要があります。
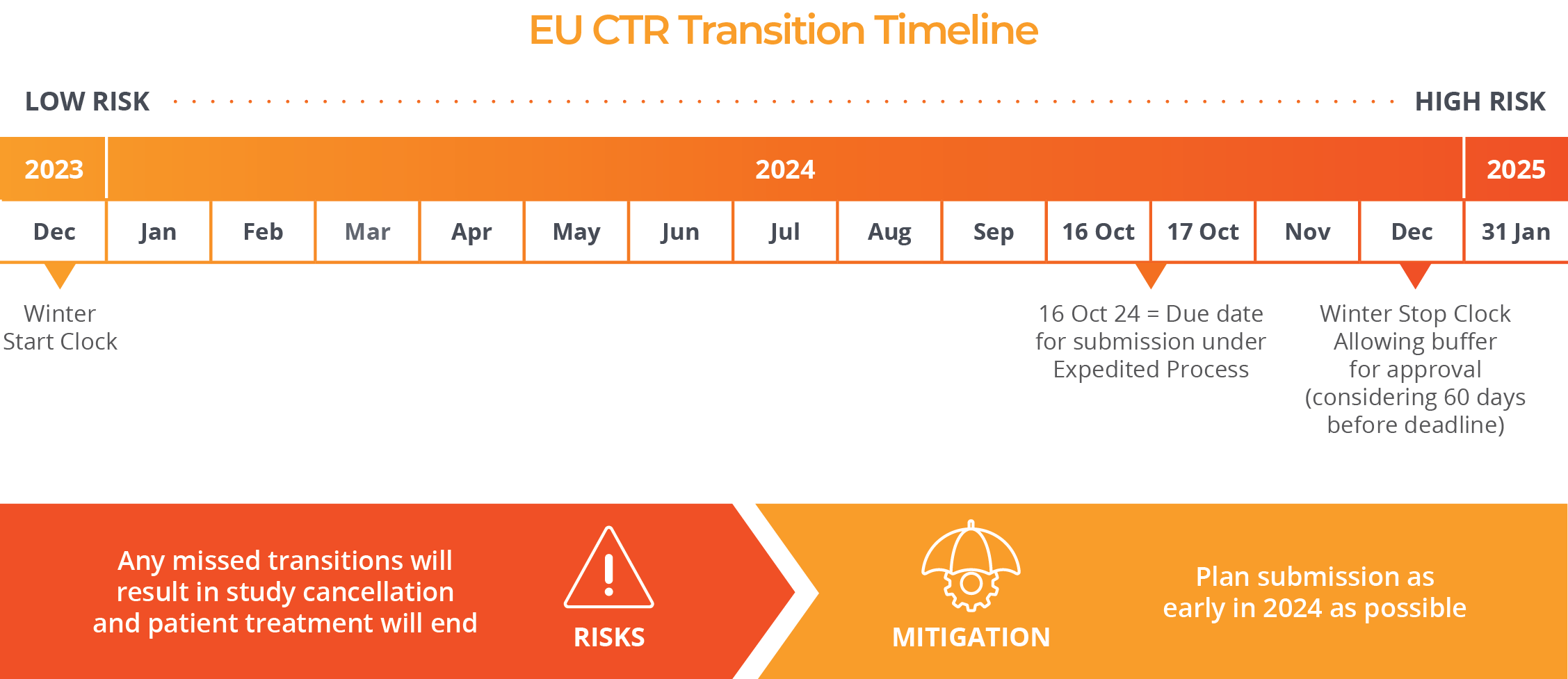
In order to transition to these new regulations, sponsors will need to submit trials before the end of the transition period on 30 January 2025. After that date, a transition will no longer be possible and a new clinical trial application under the new CTR will be required.
Until the middle of October of 2024, an administrative transition process published by the Clinical Trials Coordination Group (CTCG) is available with minimum requirements and short deadlines.
Remember, if your trial is running in EU/EEA and has at least one active site* beyond 31 January 2025, it is MANDATORY to switch the trial to the new EU regulation.
*Active site means that if the last visit last subject (LVLS) or any other trial specific intervention with the subject as specified in the protocol took place before this date, the trial does not need to be transitioned.
If there are no active sites in EU/EEA but the End of Trial has not yet been notified, the trial should not be transitioned.
Start immediately to prepare your transition.
EU CTRの目標は、参加者を最大限に守る安全基準を設け、治験情報の透明性を高めて、EUにおける臨床試験の実施に適した環境を作り出すことです。この目標を達成するために、薬事規制に新しい要件が導入されています。治験依頼企業は、規制で義務付けられた変更に合わせて対応を準備できるように、この要件を認識しておく必要があります。

The transition application is a complex procedure and includes submission using the CTR-CTIS portal of the most recently approved CTD documentation in all member states concerned. The aim of this application is for you to continue to run your clinical trial beyond 30 January 2025 without discontinuation.
Syneos Health is uniquely positioned to expedite your CTR transition journey. We have a strong, established EU regulatory framework. We also have a proven track record demonstrating our knowledge, actionable insights and quality in multiple therapeutic areas, types of products, countries and trial design applications.
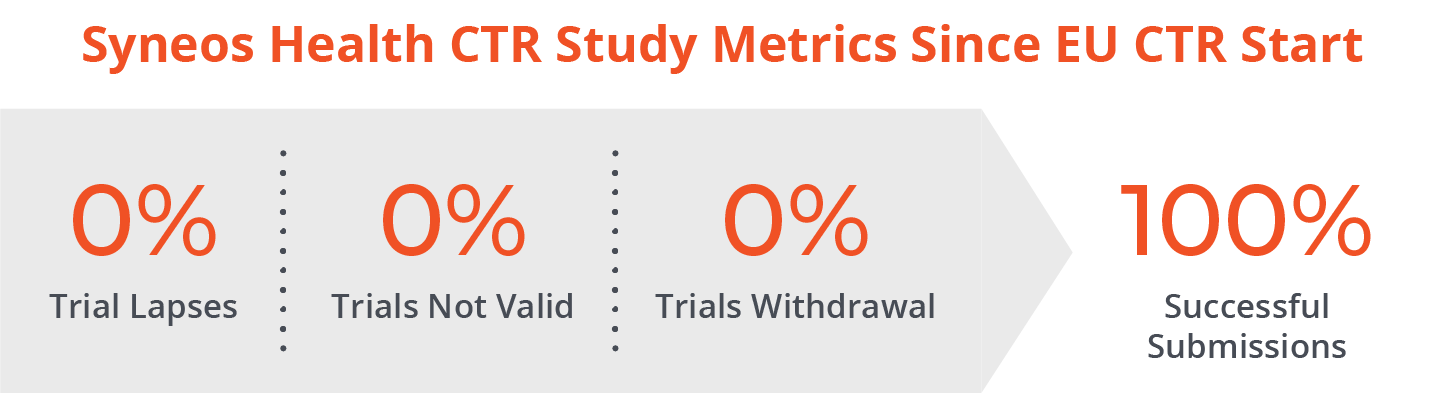
The Syneos Health integrated EU CTR offering can provide end-to-end support to sponsors irrespective of their readiness. We can support in preparing you to be ready to conduct trials under EU CTR and your ongoing delivery of clinical trials under the EU CTR. We are already supporting our customers with this.


