Patient-Powered Design: 2022 Health Trends
2022 Health Trends
In healthcare, the expectation has long been that innovation will come from a brilliant team of scientists working in a lab, while in nearly every other industry, innovation starts with a deep understanding of the end user. What happens when pharma becomes as customer-focused as other industries while maintaining that scientific integrity? A new era of higher-value research, communication and advocacy.
Patient perspectives are becoming driving forces at every stage of drug development, from clinical research to value and access. Recognizing the “patient voice” as an essential asset in the development of medicines is a relatively recent phenomenon, resulting from sustained patient advocacy, regulatory efforts and successful initiatives in early biopharma adopters.
Now, as the patient voice rises, a long-standing communications gap between patients and those who innovate on their behalf is finally beginning to close. For biopharma companies, the new focus on engagement is revealing new insights into what’s critically important to their patients in terms of research, experience and support. For patients, it’s increasing trust and overall satisfaction with healthcare.
Now, as the patient voice rises, a long-standing communications gap between patients and those who innovate on their behalf is finally beginning to close. For biopharma companies, the new focus on engagement is revealing new insights into what’s critically important to their patients in terms of research, experience and support. For patients, it’s increasing trust and overall satisfaction with healthcare.
Over the last five years or so, integration of the patient voice in all aspects of drug development has moved from experimentation to expectation. That expectation comes from both patients and regulators.
In one survey of 3,200 patients, nearly three-quarters (72%) said “trials that reflect the real world and outcomes that matter to them” are highly important and 66% said that “seeking patient input” is, too.
Learn more about how we put patient perspectives at the forefront of innovation through our Patient Voice Consortium.
Regulators are increasingly bringing patient voices into the decision-making process, specifically to understand their perspective on the disease, their current choices in treatment and what symptoms or barriers they want new treatment options to address. That involvement continues throughout development, including patient-reported outcomes and post-approval real world data collection. Most recently the FDA and the European Medicines Agency (EMA) launched a working group focused on sharing best practices in patient involvement. The FDA also began Project Patient Voice, a public database of patient-reported outcomes in cancer trials with in-market treatments.
The impact of patient-involved and patient-powered design is seen in approvals and payer negotiations, too. In fact, when patients are involved, research shows that a drug is 19% more likely to launch.
Understand how Syneos Health Value & Access can help you confidently navigate the shifting market landscape, create and communicate value, and optimize access.
Patient-Powered Engagement
How does biopharma hear and act on patient perspectives? Learning opportunities can be ad hoc or longitudinal, online or in person, live or asynchronous. Whatever the format, the strongest use cases focus on understanding patient lives / ambitions / fears, barriers to living well and accessing care, going beyond storytelling to seek advice, creating new solutions together and pressure testing every element of a trial or solution that might impact a patient before it’s launched.
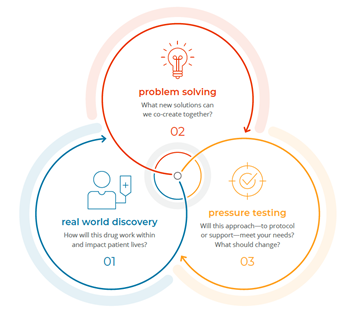
Learn more about our Real World Evidence capabilities.
The biggest driver of change in 2022 will be scale. The need for patient voices and collaboration will continue to grow and change.
1. From pilot to full operations
Many biopharma leaders have been listening to patient voices for years. But they’re entering a new era of sophistication in which engaging patients is an accountability for individuals and the organization alike.
For example, Novo Nordisk's overall approach is dubbed DEEP (Disease Experience Expert People), guided by the European Patients’ Academy on Therapeutic Innovation (EUPATI). The DEEP programs bring together patients, patient experts, patient organization representatives, and caregivers to share input on care delivery models. These programs have matured over five years to influence R&D activities and enable the patient-focused design Novo Nordisk has committed to.
2. From loud voices to lots of voices
The industry is becoming more diligent about diversifying its sources of insight. When the patient voice came primarily from secondary research, like social media listening or conference presentations, it was easy for just a handful of well-resourced individuals to have an outsized impact on the conversation, quickly becoming the leading voices for a diverse patient community. Today, organizations are connecting with a range of open and, when appropriate, private communities, advocacy and community groups to ensure that a wider number of voices are heard.
A critical element is identifying those voices and understanding and acting on the barriers created by health inequity and health literacy.
3. From qualitative to quantitative
New toolsets are making it possible to include more objective scales and measurement criteria in discussions about what and where to change. That can start with data-driven patient journeys that allow sponsors and commercial teams to see the quantitative journey of care via healthcare and claims data, creating a shared platform for discussing the qualitative impact at each step. Researchers are also sorting through disparate qualitative scales to understand what outcome measures matter most to patients. For example, Genentech and Roche worked on listening closely to patients as they develop treatments for spinal muscular atrophy. The functional scales for disease progression in that area measure everything from fine motor tasks to core functions to lower limb function, or, said another way, from fastening a button to leaning forward to walking. Their patient-powered design starts with understanding which of many measures is most meaningful to patients.
Interested in more clinical research solutions designed with a patient focus? Read more about Illingworth Research Group, a Syneos Health company.
Part of what’s driving growth in the desire to hear the patient voice are two 2022 accelerators:
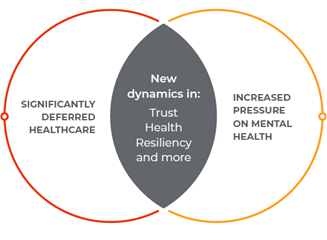
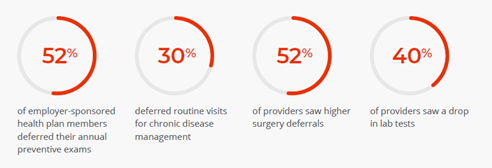
Patient support programs: A signal of opportunity
A disappointing headline rang out in mid-2021: biopharma companies spend more than $5 billion on patient support programs that only 3% of patients are using.
If you limit that data to patients who use any support program—from any source—it ticks up to 8%. The reasons are what you might expect: lack of awareness and lack of utility (personally or generally).
In 2022, data points like that one may further galvanize organizations to co-create with patients, ultimately building patient-powered experiences that earn the time and attention of the people we’re most trying to support.
Get the printable PDF of Patient-Powered Design here.
Or, subscribe now to access the full 2022 Health Trends report and our monthly email to receive exclusive updates.
References:
Lubkeman M, Lee M, Barrios G. What do patients want, and is
pharma delivering? BCG. Published June 01, 2020. Accessed November
4, 2021. https://www.bcg.com/publications/2020/what-patients-want-ispharma-
delivering
Finnegan G. New medicines 19% more likely to launch when trials are
co-designed with patients. Patient Focused Medicines Development.
Published April 24, 2019. Accessed Nov 4, 2021
Coquerel C, Kuruvilla S, Eichmann L. Inside Novo Nordisk's patient
advisory board meetings. Clinical Leader guest column. Published June 2,
2020. Accessed November 4, 2021. https://www.clinicalleader.com/doc/
inside-novo-nordisk-s-patient-advisory-board-meetings-0001
Staunton H, Trundell D. Measuring the SMA Experience. Genentech.
Published June 26, 2019. Accessed November 4, 2022. https://www.gene.
com/stories/measuring-the-sma-experience
Waddill K. Care Deferrals, Digital Tools Will Shape 2022 Medical
Cost Trend. HealthPayerIntelligence. Published June 9, 2021.
Accessed Nov 4, 2021
Phreesia Life Sciences. Expanding Awareness of Patient Support
Programs. Accessed Nov 4, 202. https://www.phreesia.com/industryperspectives-
support/


